El abordaje integral del cáncer supone hoy por hoy, un reto para los pacientes, sus familias, los equipos de salud y la sociedad. En la mayoría de los países del mundo el cáncer es la primera o segunda causa de muerte prematura sólo superada por la enfermedad cardiovascular (1). A pesar de los esfuerzos en la detección oportuna e investigación de nuevos tratamientos para los distintos tipos de cáncer aún son muchos los retos para lograr su curación (2), por esto mismo la búsqueda de herramientas terapéuticas dirigidas a la curación, remisión, control y alcanzar una mejor calidad de vida en pacientes que padecen la enfermedad esta plenamente justificada. Los diferentes productos de la colmena como la miel, veneno de abejas, polen, propóleos y jalea real han mostrado tener propiedades útiles para la prevención y tratamiento del cáncer en sus diferentes grados (3). Es precisamente sobre los mecanismos de acción de estos productos que se hablará en este artículo.
Son más de 100 los artículos de investigación que se han realizado y que directamente exploran los mecanismos de acción de los productos de la colmena en el cáncer. Buena parte de ellos se concentran en sus efectos en el cáncer de seno, próstata, colon, hígado y pulmón, todos ellos de alta carga en la población. A continuación un resumen de los principales mecanismos de acción del veneno de abejas, miel y propóleo, útiles en el tratamiento del cáncer.
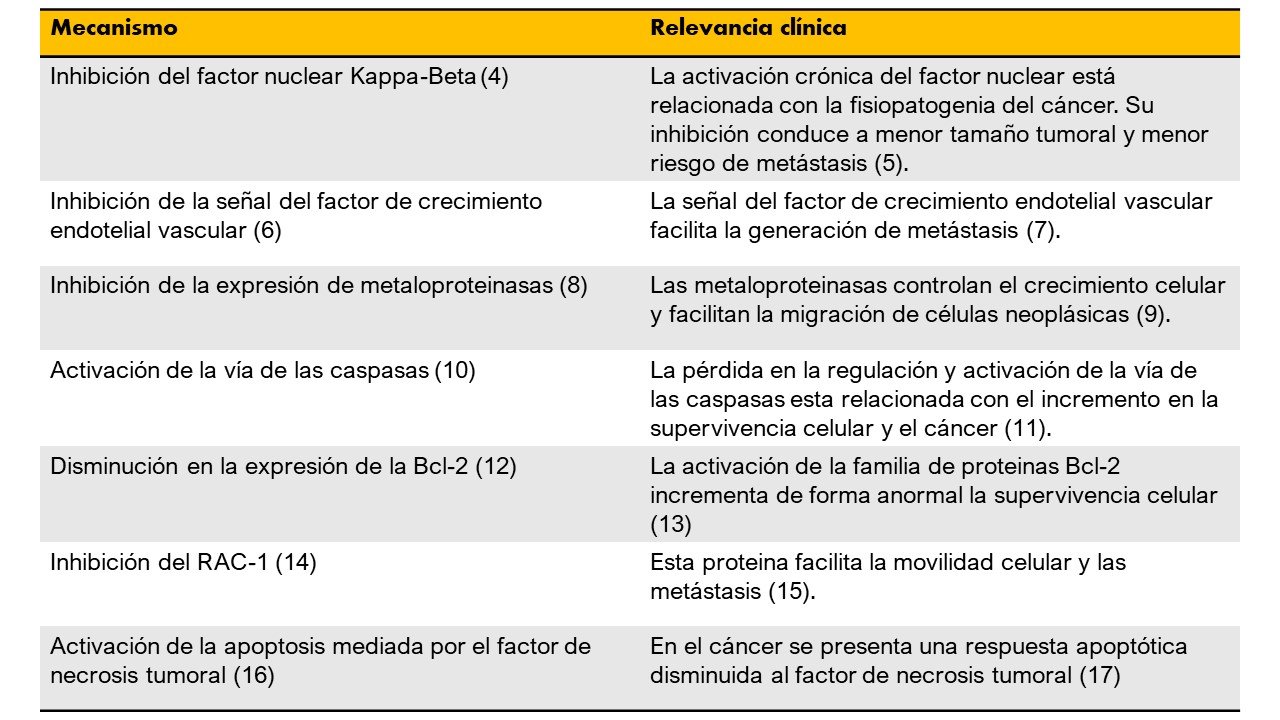
Veneno de abejas
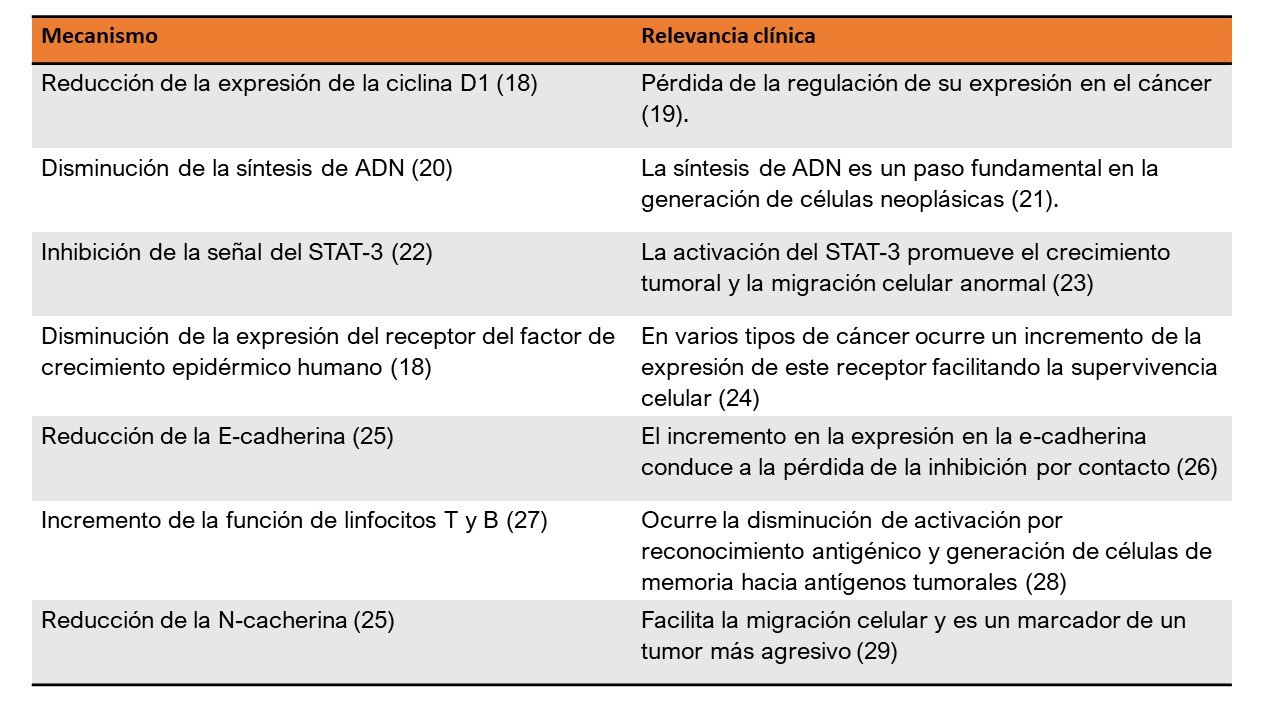
Miel de abejas
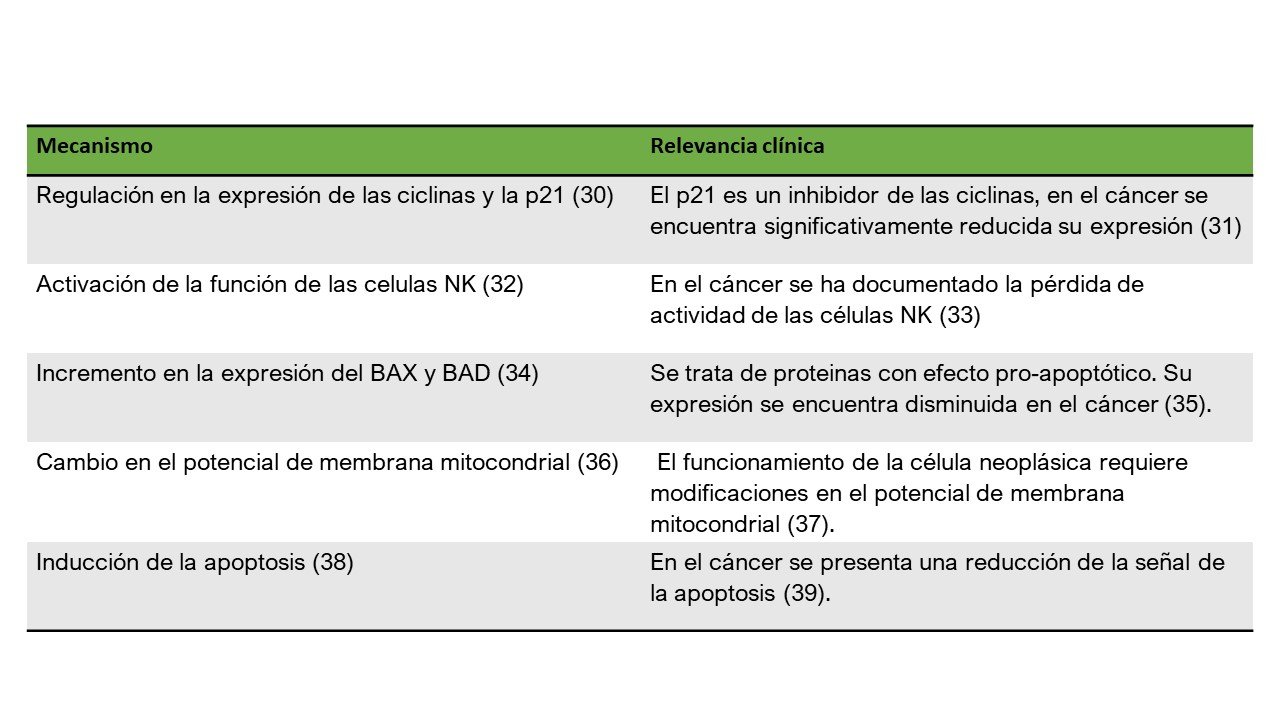
Propóleo
Referencias bibliográficas
1. World Health Organization. Global Health Estimates 2018: Disease burden by Cause, Sex, by Country and Region, 2000-2016. World Health Organization. 2018.
2. Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current Challenges in Cancer Treatment. Clin Ther [Internet]. 2016 Jul;38(7):1551–66. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0149291816301631
3. Jagua-Gualdrón A. Cáncer Y Terapéutica Con Productos De La Colmena. Revisión Sistemática De Los Estudios Experimentales. Rev la Fac Med [Internet]. 2012;60(2):79–94. Available from: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-00112012000200002&lang=pt
4. Park MH, Choi MS, Kwak DH, Oh KW, Yoon DY, Han SB, et al. Anti-cancer effect of bee venomin prostate cancer cells through activation of caspase pathway via inactivation of NF-κB. Prostate. 2011;
5. Bharti AC, Aggarwal BB. Nuclear factor-kappa B and cancer: Its role in prevention and therapy. Biochem Pharmacol. 2002;
6. Huh JE, Baek YH, Lee MH, Choi DY, Park DS, Lee JD. Bee venom inhibits tumor angiogenesis and metastasis by inhibiting tyrosine phosphorylation of VEGFR-2 in LLC-tumor-bearing mice. Cancer Lett. 2010;
7. Duda DG, Batchelor TT, Willett CG, Jain RK. VEGF-targeted cancer therapy strategies: current progress, hurdles and future prospects. Trends Mol Med. 2007;
8. Park JH, Jeong YJ, Park KK, Cho HJ, Chung IK, Min KS, et al. Melittin suppresses PMA-induced tumor cell invasion by inhibiting NF-κB and AP-1-dependent MMP-9 expression. Mol Cells. 2010;
9. Kessenbrock K, Plaks V, Werb Z. Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell. 2010.
10. Ip SW, Wei HC, Lin JP, Kuo HM, Liu KC, Hsu SC, et al. Bee venom induced cell cycle arrest and apoptosis in human cervical epidermoid carcinoma Ca Ski cells. Anticancer Res. 2008;
11. Olsson M, Zhivotovsky B. Caspases and cancer. Cell Death and Differentiation. 2011.
12. Moon DO, Park SY, Choi YH, Kim ND, Lee C, Kim GY. Melittin induces Bcl-2 and caspase-3-dependent apoptosis through downregulation of Akt phosphorylation in human leukemic U937 cells. Toxicon. 2008;
13. Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008.
14. Liu S, Yu M, He Y, Xiao L, Wang F, Song C, et al. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology. 2008;
15. Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Communication and Signaling. 2010.
16. Choi KE, Hwang CJ, Gu SM, Park MH, Kim JH, Park JH, et al. Cancer cell growth inhibitory effect of bee venom via increase of death receptor 3 expression and inactivation of NF-kappa B in NSCLC cells. Toxins (Basel). 2014;
17. Balkwill F. Tumour necrosis factor and cancer. Nature Reviews Cancer. 2009.
18. Afrin S, Giampieri F, Gasparrini M, Forbes-Hernández TY, Cianciosi D, Reboredo-Rodriguez P, et al. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 1: the suppression of cell proliferation, promotion of apoptosis and arrest of the cell cycle. Food Funct [Internet]. 2018;9(4):2145–57. Available from: http://xlink.rsc.org/?DOI=C8FO00164B
19. Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med [Internet]. 2016 Dec 2;94(12):1313–26. Available from: http://link.springer.com/10.1007/s00109-016-1475-3
20. Moskwa J, Borawska MH, Markiewicz-Zukowska R, Puscion-Jakubik A, Naliwajko SK, Socha K, et al. Polish Natural Bee Honeys Are Anti-Proliferative and Anti-Metastatic Agents in Human Glioblastoma multiforme U87MG Cell Line. Pizzo S V., editor. PLoS One [Internet]. 2014 Mar 4;9(3):e90533. Available from: https://dx.plos.org/10.1371/journal.pone.0090533
21. Zafar MK, Eoff RL. Translesion DNA Synthesis in Cancer: Molecular Mechanisms and Therapeutic Opportunities. Chem Res Toxicol [Internet]. 2017 Nov 20;30(11):1942–55. Available from: https://pubs.acs.org/doi/10.1021/acs.chemrestox.7b00157
22. Aryappalli P, Al-Qubaisi SS, Attoub S, George JA, Arafat K, Ramadi KB, et al. The IL-6/STAT3 Signaling Pathway Is an Early Target of Manuka Honey-Induced Suppression of Human Breast Cancer Cells. Front Oncol [Internet]. 2017 Aug 14;7. Available from: http://journal.frontiersin.org/article/10.3389/fonc.2017.00167/full
23. Yang Y, Liu P, Li D, Yang Q, Li B, Jiang X. Stat‐3 signaling promotes cell proliferation and metastasis of gastric cancer through PDCD4 downregulation. Kaohsiung J Med Sci [Internet]. 2019 Dec 20;kjm2.12159. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/kjm2.12159
24. Arienti C, Pignatta S, Tesei A. Epidermal Growth Factor Receptor Family and its Role in Gastric Cancer. Front Oncol [Internet]. 2019 Nov 26;9. Available from: https://www.frontiersin.org/article/10.3389/fonc.2019.01308/full
25. Afrin S, Giampieri F, Gasparrini M, Forbes-Hernández TY, Cianciosi D, Reboredo-Rodriguez P, et al. The inhibitory effect of Manuka honey on human colon cancer HCT-116 and LoVo cell growth. Part 2: Induction of oxidative stress, alteration of mitochondrial respiration and glycolysis, and suppression of metastatic ability. Food Funct [Internet]. 2018;9(4):2158–70. Available from: http://xlink.rsc.org/?DOI=C8FO00165K
26. Mendonsa AM, Na T-Y, Gumbiner BM. E-cadherin in contact inhibition and cancer. Oncogene [Internet]. 2018 Aug 21;37(35):4769–80. Available from: http://www.nature.com/articles/s41388-018-0304-2
27. Hajizadeh Maleki B, Tartibian B, Mooren FC, Krüger K, FitzGerald LZ, Chehrazi M. A randomized controlled trial examining the effects of 16 weeks of moderate-to-intensive cycling and honey supplementation on lymphocyte oxidative DNA damage and cytokine changes in male road cyclists. Cytokine [Internet]. 2016 Dec;88:222–31. Available from: https://linkinghub.elsevier.com/retrieve/pii/S104346661630521X
28. Reading JL, Gálvez-Cancino F, Swanton C, Lladser A, Peggs KS, Quezada SA. The function and dysfunction of memory CD8 + T cells in tumor immunity. Immunol Rev [Internet]. 2018 May;283(1):194–212. Available from: http://doi.wiley.com/10.1111/imr.12657
29. Mrozik KM, Blaschuk OW, Cheong CM, Zannettino ACW, Vandyke K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer [Internet]. 2018 Dec 1;18(1):939. Available from: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-018-4845-0
30. Li H, Kapur A, Yang JX, Srivastava S, McLeod DG, Paredes-Guzman JF, et al. Antiproliferation of human prostate cancer cells by ethanolic extracts of Brazilian propolis and its botanical origin. Int J Oncol [Internet]. 2007 Sep;31(3):601–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17671687
31. Parveen A, Akash MSH, Rehman K, Kyunn WW. Dual Role of p21 in the Progression of Cancer and Its Treatment. Crit Rev Eukaryot Gene Expr [Internet]. 2016;26(1):49–62. Available from: http://www.dl.begellhouse.com/journals/6dbf508d3b17c437,48fcfe1d06fc1851,5776095971b7c1e4.html
32. Takeda K, Nagamatsu K, Okumura K. A water-soluble derivative of propolis augments the cytotoxic activity of natural killer cells. J Ethnopharmacol [Internet]. 2018 May 23;218:51–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29496576
33. Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer [Internet]. 2016 Jan 23;16(1):7–19. Available from: http://www.nature.com/articles/nrc.2015.5
34. Lee Y-J, Kuo H-C, Chu C-Y, Wang C-J, Lin W-C, Tseng T-H. Involvement of tumor suppressor protein p53 and p38 MAPK in caffeic acid phenethyl ester-induced apoptosis of C6 glioma cells. Biochem Pharmacol [Internet]. 2003 Dec;66(12):2281–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0006295203006270
35. Luu TH, Bard J-M, Carbonnelle D, Chaillou C, Huvelin J-M, Bobin-Dubigeon C, et al. Lithocholic bile acid inhibits lipogenesis and induces apoptosis in breast cancer cells. Cell Oncol [Internet]. 2018 Feb 9;41(1):13–24. Available from: http://link.springer.com/10.1007/s13402-017-0353-5
36. Kimoto T, Aga M, Hino K, Koya-Miyata S, Yamamoto Y, Micallef MJ, et al. Apoptosis of human leukemia cells induced by Artepillin C, an active ingredient of Brazilian propolis. Anticancer Res [Internet]. 21(1A):221–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11299738
37. Zhang B, Wang D, Guo F, Xuan C. Mitochondrial membrane potential and reactive oxygen species in cancer stem cells. Fam Cancer [Internet]. 2015 Mar 30;14(1):19–23. Available from: http://link.springer.com/10.1007/s10689-014-9757-9
38. Aso K, Kanno S, Tadano T, Satoh S, Ishikawa M. Inhibitory Effect of Propolis on the Growth of Human Leukemia U937. Biol Pharm Bull [Internet]. 2004;27(5):727–30. Available from: http://joi.jlc.jst.go.jp/JST.JSTAGE/bpb/27.727?from=CrossRef
39. Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular Mechanisms of Apoptosis and Roles in Cancer Development and Treatment. Asian Pacific J Cancer Prev [Internet]. 2015 Apr 3;16(6):2129–44. Available from: http://koreascience.or.kr/journal/view.jsp?kj=POCPA9&py=2015&vnc=v16n6&sp=2129
